
"Having acidic pH Urine may
interfere with wide-ranging immune functions. Balancing
the pH of the body may help support a healthy immune
response and modulate systemic inflammation. "
Acidosis activates dendritic cells a
fundamental early step in any defensive immune system
response. However, if the body is constantly acidic,
that will, chronically activate dendritic cells, which
in turn
promote excessive
or hyper-reactive immune system responses and inflammation
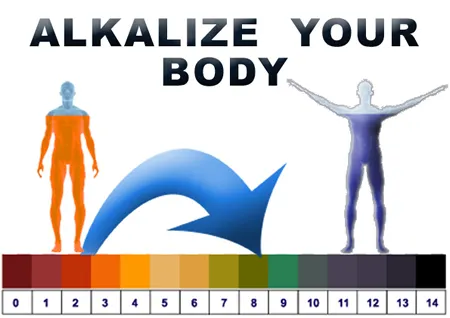
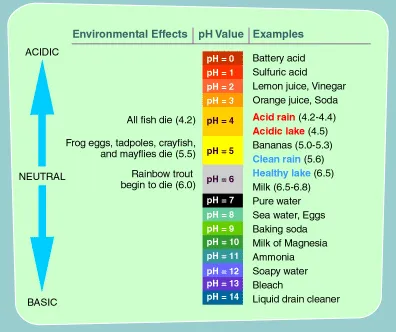
pH: What does it mean? pH is the abbreviation for potential
hydrogen. The pH of any solution is the measure of its
hydrogen-ion concentration. The higher the pH reading,
the more alkaline and oxygen rich the fluid is. The lower
the pH reading, the more acidic and oxygen deprived the
fluid is. The pH range is from 0 to 14, with 7.0 being
neutral. Anything above 7.0 is alkaline, anything below
7.0 is considered acidic.
If your urine is not alkaline 2:00 P.M.
you are definitely in an acid condition.
Acidosis is a condition in which there
is too much acid in the body fluids.
When nutritionists talk about acid-
or alkaline-forming foods, they are referring to the condition
of the food after ingestion. There are many food substances
which are acidic in their natural form that become alkaline
when broken down within the body.
| Symptoms, such as high cholesterol, hypertension
and cancer, are merely signals indicating what you
are doing wrong with your lifestyle. Suppressing
a symptom with drugs will only cover up your mistakes
and exacerbate the underlying acidity problem. Even
though conversion of acids to cholesterol can be
suppressed with drugs, more acid will accumulate,
resulting in greater likelihood to develop hypertension,
osteoporosis, cancer, etc. |
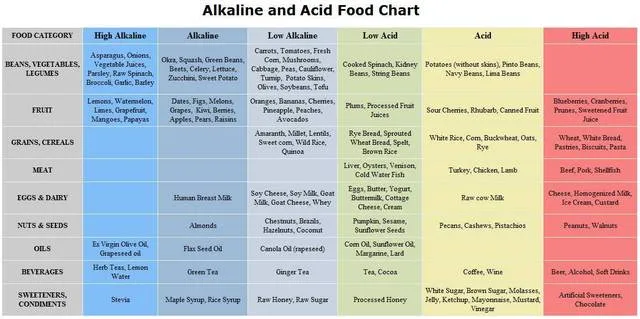
A list of Acid / Alkaline Forming Foods. >>>>more
here
Total healing of chronic illness only takes
place when and if the blood is restored to a normal,
slightly alkaline pH.
"An Imbalance In the body’s
pH may lead to serious health concerns, including:
Hormone concerns
Cardiovascular weakness
Weight gain/loss
Bladder and kidney concerns.
Immune deficiency
Acceleration of free radical damage.
Structural system weakness, including brittle bones,
hip fractures and joint discomfort
Stressed liver function
Low energy
Slow digestion and elimination
Yeast/fungal overgrowth.
Tumor growth
Breathing issues"
The body has to have a balanced pH like most living
things on earth or it does not function correctly. The
alkaline level is very important because research has
already proven that disease cannot survive in an alkaline
state and yet they thrive in an acidic environment.
Every year the number of prescriptions
written for acid-alkaline imbalances continues to increase.
Antiacids, alkalizers, specific digestive enzymes, etc.
remain popular as household "remedies" for
many acute digestive disorders. The temporary relief
experienced by these so-called remedies is interpreted
by the majority of sufferers as being a cure for the
problem. Nothing could be further from the truth. These
drugs work in much the same way as a lazy housecleaner
sweeps the dust under the rug; that is, covering up the
symptom, but not eliminating the cause.
Proton pump inhibitors (PPIs)
are frequently prescribed to treat a variety of
conditions, including gastroesophageal
reflux disease (GERD) and Helicobacter pylori infection.
Names for these drugs - lansoprazole and omeprazole,
for example - always feature the suffix "-prazole."
In 2009, they were the third most taken type of
drug in the US, and the Food and Drug Administration
(FDA) estimates 1 in 14 Americans have used them.
Over time, however, experts have begun to question
the safety of the drug.
Experts initially believed that use of PPIs was
only risky for patients with coronary artery disease
who were also using the antiplatelet drug clopidogrel,
considering the risk to be caused by a drug-drug
interaction. More recent studies, however, have
indicated that the risk may extend further.
"Our earlier work identified
that the PPIs can adversely affect the endothelium,
the Teflon-like
lining of the blood vessels," reports senior
author Dr. John Cooke. "That observation led
us to hypothesize that anyone taking PPIs may be
at greater risk for heart attack." Source |
Our bodies are like the rug in that they
will only allow the drugs to cover up the problem for
so long. Eventually, these acute digestive disorders
will become chronic, resulting in a more difficult condition
for the body to deal with. So, what was once simply a
minor case of acid indigestion or heartburn becomes a
major digestive ailment. The stomach, liver, small and
large intestines, kidneys and pancreas can all be seriously
impaired, both from consumption of an improper diet and
from the use of drugs that cover up an overly-acidic
diet and the, consequent indigestion.

Almost anyone who has been eating the standard
diet of meat, dairy foods and refined and processed foods
will suffer in varying degrees from an acid-alkaline
imbalance. Add to this fare: alcohol, cigarettes, drugs
and condiments, and the percentages will rise even higher.
The physicist MANFRED VON ARDENNE ... recently
discovered that cancer cells owing to their fermentation,
are more acid - inside and on their surface -
than normal cells ... |
When people encourage you to "alkalize
your blood," most of them mean that you should eat
plenty of foods that have an alkaline-forming effect
on your system. The reason for making this suggestion
is that the vast majority of highly processed foods -
like white flour products and white sugar - have an acid-forming
effect on your system, and if you spend years eating
a poor diet that is mainly acid-forming, you will overwork
some of the buffering systems mentioned above to a point
where you could create undesirable changes in your health.
Keep in mind the following basic concepts:
Organic matter is taken into the body in
the form of food.
This organic matter is broken down into
simple compounds (monosaccharides, amino acids, lipids
etc).
After metabolism, these compounds leave
an acidic or alkaline residue in the body.
The simple compounds contain elements such
as sulphur, potassium, sodium, magnesium and calcium.
These minerals determine the H+ concentration and thus
the acidity or alkalinity of the body.
These elements are either acid-forming elements or alkaline-forming
elements. The acid-forming substances are sulphur, phosphorus and chlorine,
while the alkaline formers are sodium, potassium, calcium, magnesium
and iron.
Within the plant kingdom, the organic acids
found in fruits and vegetables are metabolized and eventually
become carbon dioxide and water. The alkaline elements
such as potassium, calcium, sodium and magnesium remain. Although many fruits are acidic in nature, when broken
down into their constituent elements, the acids are rendered
neutral and the alkaline elements are dominant. Therefore,
the end result of the organic breakdown and digestion
of fruits and vegetables is alkaline in nature.
"There are many studies from
around the world that show that the more protein
a society consumes,
the more osteoporosis they have. Osteoporosis
is definitely an acid disease. The calcium is
just
leached out of the bones by these metabolic acids.
Calcium, or lack thereof, is not the problem,
over acidification is. This calcium has to be
excreted
by the kidneys or in the feces or it will be
deposited, somewhere in the body. It can be measured
in the
urine. It can be deposited in the lining of the
arteries, kidney or gallbladder stones can develop.
It can be deposited in the brain causing dementia or arthritic
deposits form, on and on...and then the microbes come out of
the acidic, hurt, swollen cells to help get rid of the deposits.
Inflammation
develops, pain, more swelling, blocked arteries. The amount of
calcification in the body correlates directly with the onset
of 'old age'. Also, all these old micro-organisms are being re-discovered,
inside the diseased tissues effected by Chronic Degenerative
Disease,
cancer, the bacteria Chlamydia pheumoniae being isolated from
the arteries in most cases of hardening of the arteries." |
THE PH/DIGESTION/ELIMINATION AXIS 1997
by Jim LeBeau Perfect Health Foundation
The pH/digestion/elimination axis is so
basic to human health that many highly educated “experts” in
nutrition pass it right by as they endlessly complicate
and confuse things. Read the following carefully, taken
from page 496 of ANATOMY AND PHYSIOLOGY by Anthony and
Thibodeau, 10th edition (a college level text) ¼
“enzymes FUNCTION OPTIMALLY AT A
SPECIFIC pH and become inactive if this deviates beyond
narrow limits”.
“enzymes are vital substances. Without
them, the chemical reactions necessary for life could
not take place”.
Click piture for larger
IN OTHER WORDS .
without enzymes we could not live. We could
not digest anything, or metabolize anything, or even
breathe right. We would die with minutes. without the
right pH balance in saliva the enzyme amylase that begins
the digestion of starch . . . the enzyme that turns that
bite of bread sweeter and sweeter as you chew it . .
. cannot work right . . . without the right pH balance
of stomach juices (acid) the protein you just swallowed
will not get properly broken down and your stomach enzymes
will not work right either- without the right pH balance
of bile and pancreatic juices (alkaline) where enzymes
complete the digestion of fats, carbohydrates, and proteins,
not one of the millions o workhorse enzymes in those
digestive juices can cork right either. all of
the above means that all of the best and most nutritious
food in the world and the most expensive vitamin pills
and supplements won’t get digested and assimilated
right if your body pH is off balance .
.and it also means that YOU cannot work right either.
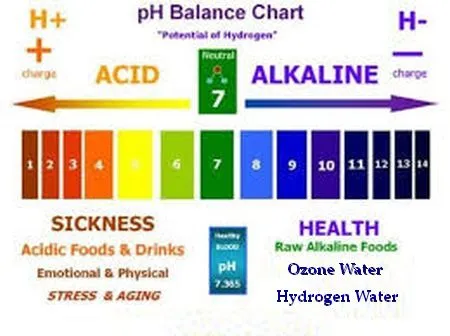
It's important to note that on the pH scale,
each number represents a tenfold difference from
adjacent numbers; in other words, a liquid that
has a pH of 6 is ten times more acidic than a liquid
that has a pH of 7, and a liquid with a pH of 5
is one hundred times more acidic than pure water.
Most carbonated soft drinks (pop) have a pH of
about 3, making them about ten thousand times more
acidic than pure water. Please remember this the
next time you think about drinking a can of pop. |
HOW WE BECOME
ACID
How acid something is determined by measuring
its pH. The pH of anything is set on a scale of from
1 to 14. pH 1 is the most acid, like the acid in your
car battery. pH 14 is the most basic, like the lye you
spray in an oven to clean it. Water is supposed to be
neutral at a pH of 7.0. The pH of the blood has to remain
exactly 7.40, all the time...exactly.
If the blood's pH rises or falls one tenth
of a pH unit you are in intensive care in the hospital
where the pH of your blood is monitored very carefully.
If it moves two tenths either way it is lethal. How the
blood always maintains a constant pH is a very complex
matter and one that everything in the body helps to maintain,
as everything in the body depends on this sameness.
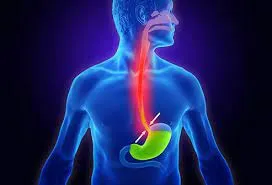
Acid Reflux is caused by to much Acid,,,The backflow
of stomach acid and digestive enzymes (pepsin) can
wreak havoc on your esophagus (the food passage that
goes from your throat to your stomach), as well as
your ears, nose, throat, vocal cords, sinuses, mouth,
and lungs. Pepsin, in the presence of acid, digests
protein and damages tissue. Outside the protected
stomach, pepsin, bathed in acid, digests you! And
when pepsin attacks your sensitive airway and esophageal
tissues, you can suffer all kinds of problems. The
most common reflux symptoms are hoarseness, chronic
cough, throat-clearing, post-nasal drip, sinusitis,
sore or burning throat, difficulty swallowing, shortness
of breath, snoring, sleep apnea, bad breath, tooth
decay, asthma, and COPD. Unfortunately, your doctor
is probably unaware that these symptoms may be caused
by acid reflux and that it could be controlled with
an alkaline water and food protocol. |
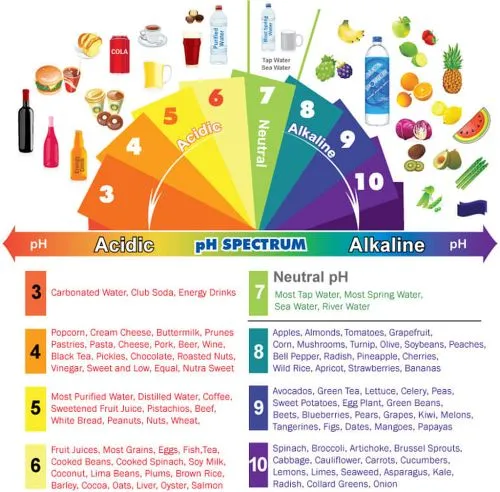
Strong Acids, Weak Acids and Protein
The strong acids in our bodies are those that
are formed by the degradation of protein. These are sulfuric
acid, phosphoric acid and nitric acid. These are strong,
like the battery acid in your car. Strong acids are strong
in contradistinction to weak acids such as vinegar and
citrus juices. Weak acids do not ionize (break apart
completely) when in solution whereas strong acids do.
This is why vinegar does not burn holes in your clothes,
or dissolve your bones; it does not break apart completely
into an acid and a base part, it remains partly a salt.
A salt is formed when an acid and a base combine and
neutralized each other.
The Main Reason We Become Acid
Is From Over-consumption Of Protein
When protein breaks down in our bodies, it
breaks down into the above mentioned strong acids. These
three acids must be excreted by the kidneys because they
contain sulfur, phosphorus or nitrogen which cannot break
down into water and carbon dioxide to be eliminated as
the weak acids are. In their passage through the kidneys
these strong acids must take a basic mineral with them
because in this way they are converted into their neutral
salts and don't burn the kidneys on their way out as
would happen if these acids were excreted in their free
acid form. Sulfuric acid or any of the strong acids are
excreted mainly as the salts of sodium, potassium, magnesium
or calcium as these are the main basic minerals of the
body, the ones that are the most plentiful. The sulfur
in sulfuric acid can and does combine with the calcium
in your bones for one and is excreted as the corresponding
salt which is called calcium sulfate. This salt does
not harm the kidneys on its way through them but it does
rob the body of the needed basic calcium.
We need protein, obviously, but all we
need is 40 grams a day, a training athlete may need 80
grams a day. The average American diet on the
other hand contains as much as 200 grams of protein per
day, that's
bacon and eggs for breakfast, etc.. We all know that
the "richer" we became as a civilization and
more " advanced", the more meat we eat.
Respiratory rate and breath holding ability
are key indicators of the pH of blood. Why? The
body uses carbon dioxide levels as a primary way
to regulate blood pH. When you dissolve carbon
dioxide in the blood, you get carbonic acid. If
there is too much carbonic acid, the respiratory
center in the brain will send signals to the respiratory
muscles that they must breathe to get rid of excess
carbon dioxide. Respiratory rate will be rapid
- often more than 18. The breath hold time will
be short. This is an acidosis situation.
If there is too little carbonic acid, the
respiratory center will send out a message
to reduce respiration to conserve carbon dioxide.
The respiratory rate may drop to less than
12 and the person will be able to hold their
breath a long time. This is an alkalosis situation.
A person with an acid imbalance will generally
have a resting respiratory rate of 19 or more
and if they take a deep breath will only be
able to hold it for 40 seconds or less. Why?
Since the blood is acid, the brain tells the
body to breathe faster to get rid of the acidic
carbon dioxide. The same principle applies
to breath hold. The brain sends messages to
breathe to get rid of excess carbon dioxide.
The urine pH may be acid. But if the acidosis
is caused by excess potassium in the body,
the kidneys will dump potassium (alkaline)
and retain hydrogen (acid). So the urine is
alkaline while the blood is acid! Source |
There is also a daily rhythm to this acid-base,
ebb and flow called by Friedrich Sander the Base Flood
and Base Tide. The stored acids are mobilized from the
connective tissues and Pishinger's Space while we sleep.
The space enclosed by these finer and finer
fibers, is called PISHINGER'S SPACE, from the German
scientist that described it. Essentially, this
is the extracellular space that contains the fluids
that bathe and feed each and every cell while carrying
away the wastes from those same cells. There is
no mention of this organ in American, physiology
text books, there is the extracellular space . |
These acids reach their maximum (base tide)
concentration in this fluid, and thereby the urine, at
2:00 AM, so the urine is the most acid at this time.
The acid content of the urine directly reflects the acid
content of the fluid in Pishinger's Space, the extracellular
fluid compartment of the body.
Click picture for larger view
Your
PH
By the time you get up though, in the morning,
all the acids consumed and generated the day before should
be gone, excreted while you slept, contained in your
bladder and ready to be voided when you wake. This first
urine should be acid when you get up in the A.M.. The
urine pH you should check though is the pH of the urine
measured the second time you empty your bladder in the
morning as this reflects the pH of the body fluids at
that time, in the morning, not the pH of the urine from
the night before. Therefore, your A.M. urine, the second
voided specimen after you get up, should be back to about
neutral, close to pH 7.00 (pH 6.8 to be exact). Because
most everyone is acid, this is hardly ever the case.
More and more acids accumulate day after day and chronic,
degenerative disease develops as the direct result of
the pleomorphic changes that take place in the blood
as discussed above. Each day we add to the acids not
disposed of the day before. On the other hand, this Pishinger's
Space, becomes most alkaline around 2:00 PM, the Base
Flood, as then the most bicarbonate is being generated
by the cover cells of the stomach (see below), after
lunch and breakfast have been metabolized, actually.
If your urine is not alkaline at 2:00 P.M. you are definitely
in an acid condition.
In the normal situation, hydrochloric acid
is produced by the cover cells of the stomach. Table
salt, sodium chloride, is split into hydrochloric acid
and sodium bicarbonate. The production of each molecule
of hydrochloric acid is matched by the production of
an equivalent molecule of sodium bicarbonate The acid
goes into the stomach and and the sodium bicarbonate
goes into the blood stream and circulates all around,
first flushing out the excess acid in the tissues and
especially, freeing the collagen fibers and the colloidal
connective tissue organ from the adsorbed acids stored
there. Any bicarb that is left over, is picked up by
the alkaline glands, the liver, pancreas, etc..
Lemons and Limes One of the interesting facts is that lemons
and limes are alkaline forming. Although they
are acid by nature, in the body they are alkaline
forming because of their mineral content. This
makes them ideal for salads and other meal items
when you need an acid taste component. You will
see in the list that vinegar is rated very acid
at (-39) while lemon is alkaline at (+9).
|
An imbalance happens, of course, if enough
alkaline food is not eaten and because the sodium bicarbonate
generated by the stomach's cover cells, does not all
go to the alkaline glands (pancreas, liver, salivary
glands and the alkaline glands in the duodenum). On the
way through the body to those glands, some of it gets
used up by neutralizing acid residues from the previous
meal and ones stored in the connective tissue organ from
before.
A
list of Acid / Alkaline Forming Foods

If there is not enough base left over after
a meal, enough base to neutralize and clear the acids
stored in the connective tissues,
a relative base deficiency develops which is again, the latent "acidosis".
When this happens the liver and pancreas don't end up with enough alkaline
juices to ensure proper digestion. Digestion can't proceed without
enough of these alkaline juices for the liver and pancreas, etc., so
the stomach has to produce more acid, in order to make enough base,
ad nauseam, and one can develop stomach ulcers. The ulcer is not
the result of too much acid, on the contrary, it is the result of too
little base!
So, in this acid condition we are talking
about, we aren't "acidotic" in so many words,
rather we are base deficient. This is why 80 or 90 year
old, old folks, are shrunk up, little people. They have
no mineral stores left. When all the minerals are gone,
so are we, our battery runs down.
It is just like a battery. The cells of
our body do carry a charge that can be measured as the
oxidation/reduction potential of the blood. This energy
potential decreases with aging, just as the minerals
do. We become more oxidized (so the need for antioxidants).
Both things occur because of hyper-proteinization, too
much protein.
We aren't acidotic as they say in a hospital,
in shock, when things have gone so bad that the very
pH of the blood itself begins to change, Code Blue. Rather,
in a state of latent "acidosis" we are full
of stored acid residues, residues stored in the Pishinger
space waiting for a ride out on base minerals that aren't
there. This is the latent in latent "acidosis".
Blood values have not started to change yet, so the acidosis
is stored in the tissues as it were. The tissues are
acid but technically this is not an acidosis either as
the blood appears normal.
If things get worse, this latent "acidosis" can proceed into
what is called a compensated acidosis. This means the blood pH itself
still hasn't started to change but other values in the blood have had
to change to keep the blood pH the same 7.40 that it is supposed to
be. Decompensated acidosis is when the blood pH itself is effected.
CAUSE OF CANCER & pH
Herman Aihara, in his book entitled “Acid & Alkaline”,
states that:
If the condition of our extra
cellular fluids, especially the blood, becomes
acidic, our physical condition will first manifest
tiredness, proneness to catching colds, etc.
When these fluids become more acidic, our condition
then manifests pains and suffering such as
headaches, chest pains, stomach aches, etc.
According to Keiichi Morishita in his Hidden
Truth of Cancer, If the Blood develops a more
acidic condition, then our body inevitably
deposits these excess acidic substances in
some area of the body such so that the blood
will not be able to maintain an alkaline condition
which causes these areas such as the cells
to become acidic and lowers in oxygen.
As this tendency continues,
such areas increase in acidity and some cells
die; then these dead cells themselves turn
into acids. However, some other cells may adapt
in that environment. In other words, instead
of dying - as normal cells do in an acid environment
- some cells survive by becoming abnormal cells.
These abnormal cells are called malignant cells.
Malignant cells do not correspond with brain
function nor with our own DNS memory code.
Therefore, malignant cells grow indefinitely
and without order. This is cancer. |
if the pH of the intestines is not right,
different bacteria and eventually yeast can grow there,
dysbiosis (wrong growth), in place of the bacteria that
should be there. This causes its own set of problems.
If the environment of the intestines is not alkaline but acid, dysbiosis
(wrong growth) occurs. The gut fills with and supports the growth of
the wrong kind of bacteria, fungus, yeast, Candida sp., etc.. These
bacteria in turn generate their own acidic, toxic byproducts of metabolism
that further aggravate and maintain the already latent "acidotic" condition.
When this dys-biosis or wrong growth begins, it begins with fermentation
and as fermentation is the process of eating, metabolizing and excreting
that bacteria do, alcohol is produced. Fermentation like this can even
cause cirrhosis of the liver in patients that have never drunk alcohol
in their life. As when making wine, this fermentation process can go
'bad' and begin to rot. Vinegar and other rotten things are produced.
This vinegar acid and the other things can cause "heart burn" too,
along with the bloating and gas that come with the fermentation process
but this kind of heart burn is not from too much acid, hydrochloric
acid, it is from not enough.
In this kind of heart burn, that comes
an hour or two after you eat, other acids form, acetic
acid as in vinegar and other putrefactive acids. These
acids cause the "heart" burn. The meal is not
digesting well as with a good amount of hydrochloric
acid, it is fermenting instead. These rotten things are
reabsorbed back into the body and picked up by the blood
like anything in the gut. These rotting things in the
gut just don't make you feel well. It's why there are
constipation headaches, sleepless nights from food eaten
too late to digest (nights where undigested food just
ferments and rots all night, makes bad dreams). The skin
also tries to expel such toxins, pimples, rashes and
other skin problems develop.
With this kind of "heart burn" one
hurts after eating, right away or an hour or two later,
rather than before as with an ulcer. This can burn with
reflux up the esophagus, worse while lying down, or it
can be just pressure over the whole abdomen from the
gas. This gas can actually push the stomach through the
diaphragm into the lung cavity, producing a hiatal hernia.
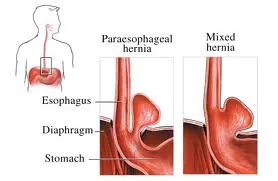
The
Symptoms of Being Acid
The acidity, the pH of the body, it's
fluids and cells, is the most important homeostatic or
balancing act the body has to perform. The acidity of
the blood has to remain exactly the same all the time.
The fact that we are alkaline beings by design but acid
generating beings by function makes this the most basic
function the body has to perform, no pun intended, besides
and including breathing and pumping one's blood around.
As we become more and more acid, accumulate and store
more acids in our connective tissues this is what happens;
A. First, there is an increased sense of well being from the "stimulatory" reaction
of the bodies regulatory system that operates in high gear to
process the excess acid.
B. The patient therefore believes her or his self to be totally well.
C. This type of person tends to be an over achiever, active, always
running.
D. The person is overly ambitious due to the acidic irritation of the
nerves.
E. Later, as the process progresses the patient becomes;
1. irritable and difficult to please
2. exhausted, fatigued
3. listless and inability to get anything done
4. constantly finds fault
5. sees only the pessimistic side of life
6. can't sleep restfully
7. wakes tired in the A.M.
8. generalized aches and pains
9. I. loss of appetite or ravenous hunger
10.J. obstipation (difficulty moving bowls) to constipation gallbladder
pains and frequent headaches
11.frequent redness of the nose or parts of the nose
12.hardness and pain of the neck and shoulder muscles with pressure,
and pain of the back of the head nerves with pressure
13.often coated tongue and halitosis, enlarged tonsils
14.moist hands with poor blood supply, cold hands, pale to white
15.tendency to sweat, tendency to development of skin rashes
16.susceptibility to colds and bronchitis with large mucous secretions
as an attempt to rid the body of acid, the excretion and reaction
phases of Homotoxicology
17.women tend to be pale with scant, heavy or irregular periods
18.blood pressure tends to be lower at first
19.The Indicin-Test of the Urine (see below) is usually positive. This
is a test for rotten products in the intestine that are reabsorbed
by the
blood stream and re-excreted out the urine when the intestines are
in a dysbiotic condition, when abnormal bacteria are growing there
because of the latent acidosis
20.shows aging as the sodium is depleted from the body fluids and potassium
from muscles causing wasting and weakness, and then
calcium from the bones which is osteoporosis, arthritis and the like.
|
Top of the Line
Waterproof pH Meter
Ideal for all pH testing, water
purification applications, wastewater regulation,
aquaculture, hydroponics, colloidal silver, labs & scientific
testing, pools & spas, .
FEATURES
• Measures pH and Temperature
• Auto-ranging three point calibration with digital fine tuning
• Includes storage solution in a sponge embedded in a clear cap
• Waterproof housing (IP-67 rating)
• Simultaneous temperature display
• Measurement Range: 0-14 pH
• Digital automatic calibration (one point), with digital fine tuning
• Automatic Temperature Compensation (ATC)
• Auto-off function, data-hold function and low-battery indicator
• Display: large and easy-to-read LCD screen includes simultaneous temperature
reading
• Factory Calibrated: The PH-200 meter is three-point checked and factory
calibrated to pH 7.0. It can be re-calibrated to any point in its range with
digital calibration using the push buttons.
More Information
|
Testing Your PH :
1. Saliva test upon waking. First thing in the morning
right when you get out of bed, lick the end of you
ph meter with saliva. Note down
that pH number. Do this before brushing your teeth, drinking, smoking,
or even thinking of eating any food. This pH should be 6.8.
2. Then test your second urine of the
morning. The urine stored in your bladder during the
night, that is ready to be eliminated when you get up,
should be acid so you don't want to test that. Drain
your bladder in the morning, the last time you get up
if you get up during the night and then see what that
urine pH is. Again, record this number. This number should
be the pH of your urine after you got rid of your acid
load from the day before. The acids should be gone the
second time you go to the bathroom so your urine pH should
be around 6.8 also.
3. Eat breakfast, an apple will do, anything,
and five minutes after breakfast check your saliva again.
Write this number down also. This
number should go up from what it was before you ate, the more the better.
4. and 5. Then check your urine pH between
meals, i.e. between breakfast and lunch and between lunch
and dinner. The pH should always be 7.0 to 8.5, a couple
of hours after meals.
These five
tests show the following:
1. How well your digestive system dealt
with what you ate the night before, i.e. the AM urine
pH. These numbers may change from day to day depending
on what you did eat the night before.
2. How well we treat ourselves in general, i.e. how "strong" the
liver is. This is the AM saliva pH. This number shows the overall state
of our health, the condition of the alkaline reserve of our bodies
which reflects the diet we have eaten over the last months to years.
This number stays rather constant and will only change after some work
has been done in re-mineralizing the body.
Since the saliva pH is an indicator of
intracellular pH, saliva pH readings should never be
below the pK of the phosphate buffer
system, 6.8. . The most accurate reading of saliva pH is recorded immediately
upon awakening--after sleeping at least five hours and before brushing
the teeth. It is during sleep that the body removes waste and is in
an anabolic state restoring and replenishing the body.
If the patient has a saliva pH of 5.5
at this time and only 5.6 after eating, you know that
this person has no alkaline reserve and that his body
is devoid of the minerals necessary to process food properly--his
body cannot adequately respond to the physiological crisis
of handling food.

3. The pH of your saliva after you eat
gives an indication of what the mineral reserves of your
body are (the pH number should increase
after you eat). My son just thought of a lemon for a minute and the
pH of his saliva went up a whole point. He had enough reserve
minerals, which are basic, to pull into his digestive system to begin
the digestive process.

The ideal saliva pH pattern is 6.8 on
awakening, 7.0 before eating and 8.5 following breakfast.
Besides just thinking of a lemon one can eat one. This
is a simple test that can be done at most any time of
the day. It too checks the adequacy of the alkaline reserve
of the body. When a healthy person with adequate alkaline
reserves takes a bite of highly acid lemon, the saliva
pH drops sharply for an instant but returns almost immediately
to pH 8.5. The more acidic the food that is eaten, the
more rapid the response of the alkaline reserve, and
the higher the saliva pH should be following a meal.
4. The pH's of the urine between meals
should be kept in the basic range, pH 7.0 to 8.5. After
one eats, the stomach generates the necessary acid to
digest the food. While doing this, it also performs the
opposite action, i.e. it makes an equivalent amount of
base or stream and delivered to the alkaline glands of
the body, the saliva, the pancreas and the liver. The
maximum amount of base in the blood and therefore in
the urine occurs one to two hours after you eat.

This rhythm of the acid and base flow
of the body, is called by Frederick F Sander, the Base-floods
and the Base-tides of the Acid-Base household. This information
is from, The Acid-Base Household of the Human Organism
and its cooperation with the nail circulation and the
rhythm of the Liver, Frederick F. Sander, about 1930,
translated from the German by Robert Miller, D.C. This
book is not yet in
print in English.
Actually the body fluids and therefore
the urine is most acid at 2:00 A.M. (pH 5.0 to 6.8) in
the morning (the base tide) and most alkaline at 2:00
P.M. (pH 7.0 to 8.5) in the afternoon (base flood)." The
ideal pH numbers depend on the time of day. Plotted on
a curve it looks like the double hump of the back of
a camel. Two times a day the urine should be alkaline
and that is the top of the humps and corresponds to 10
A.M. and 2 P.M., the alkaline tide after meals. During
the rest of the day the pH should be between 6.6 and
6.8. This is optimal urine. The first urine in the morning
should be more acidic because of the decalcification
that takes place during the night."
If all the acids are not all flushed out during the night they accumulate,
day after day. The cycle of chronic disease begins. It effects
different people in different ways; heart disease in one, arthritis,
osteoporosis, stones, ulcers, cancer, in others.
If what you are doing to get better
isn't working, if you are sick, be it with modern allopathic
medicines or any of the alternative, complementary
therapies, it is probably because you haven't dealt
with this acid problem, first.
THE
TREATMENT OF BEING ACID
Source

MONITOR
YOUR "PH" DAILY



Large LCD displays PH or mV and Temperature
simultaneously. The backlight starts automatically after
power on for easy viewing at day or night. Measures PH
range 0-14 with 0.01 resolution. Highly accurate for
up to ±0.05PH variance
The product is convenient to use and simple to operate
with high measurement precision and is Suitable for
Homes, schools & colleges,
laboratories, pharmacy, etc
 |
Digital PH Meter with ATC Water PH Test Meter with
0-14 ph Measure Range High Accuracy 0.01 PH Pen Tester
LCD display: 3 digits and a half digital display
PH
range: 0PH~14PH
mV range: -415mV~415mV
Resolution: 0.01PH, 1mV
PH measurement error: ±0.05PH
mV value measurement
error: ±0.1% FS
Temperature of the tested solution:
5~60°C
Temperature compensation range: 0~60°C
Automatic
shutdown time: four minutes
Easy to Use
Package includes:
1 x Digital pH Meter (Battery not included)
2 x Carry Case
3 x Instructions
|
PH
Meter
To Receive these Amazing free newsletters click here
|
Top of the Line Waterproof pH Meter
Ideal for all pH testing, water purification applications,
wastewater regulation, aquaculture, hydroponics,
colloidal silver, labs & scientific testing,
pools & spas, .
FEATURES
•
Measures pH and Temperature
•
Auto-ranging three point calibration with digital
fine tuning
•
Includes storage solution in a sponge embedded
in a clear cap
•
Waterproof housing (IP-67 rating)
•
Simultaneous temperature display
•
Measurement Range: 0-14 pH
•
Digital automatic calibration (one point), with
digital fine tuning
•
Automatic Temperature Compensation (ATC)
•
Auto-off function, data-hold function and low-battery
indicator
•
Display: large and easy-to-read LCD screen includes
simultaneous temperature reading
•
Factory Calibrated: The PH-200 meter is three-point
checked and factory calibrated to pH 7.0. It can
be re-calibrated to any point in its range with
digital calibration using the push buttons.
More
Information
|
How
much water do you need?
The equivalent of 8 cups of water
for women and 12 cups of water for men is the minimum
amount of fluid recommended daily to replace water
losses under conditions of moderate activity, mild
temperature and altitude |
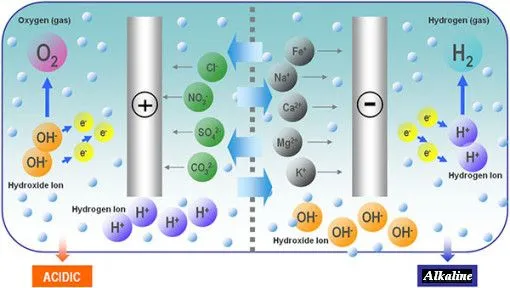
Can YOU Benefit from Hydrogen Water
The
Ultimate Anti-Oxidant?
Make
Hydrogen Water for the Whole Family

