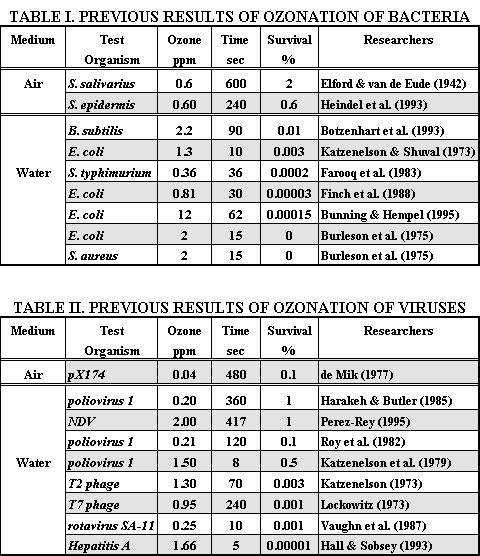
|
OZONE SCIENCE & ENGINEERING 0191-9512/98 $3.00+.00 Bactericidal
Effects of High Airborne Ozone Concentrations by W. J. Kowalski1, W. P. Bahnfleth1, and T. S. Whittam2
The Pennsylvania State University
Abstract The bactericidal effects of high concentrations of airborne ozone were tested against Escherichia coli and Staphylococcus aureus. Petri dishes containing the microorganisms were inserted in a chamber and exposed for 10 - 480 seconds to ozone concentrations between 300 and 1500 ppm. Death rates in excess of 99.99% were achieved for both species. The mechanism of inactivation accorded with the predictions of first and second-order kinetics, suggesting that the disinfection action of ozone in air parallels the action of ozone in water. Introduction The transmission of respiratory infections in indoor environments represents a major public health concern for which engineering alternatives are limited. Evidence for the dissemination of respiratory diseases inside buildings, and specifically by ventilation systems, exists in the epidemiological data (Hers and Winkler, 1973; Williams, 1960; Zeterberg, 1973; Riley and O'Grady, 1961). Common technologies, such as high efficiency particulate air (HEPA) filters and ultraviolet germicidal irradiation (UVGI), have proven less effective in real-world installations than in laboratory studies (Weinstein, 1991; Miller-Leiden et al., 1996) and have inherent limitations. New disinfection options are needed for building designers, and the success of ozone in water systems suggests its potential for airside applications. This study seeks to determine if airborne ozone can be used to rapidly inactivate pathogenic agents, with the long-term goal of applying this method to the disinfection of ventilation system airstreams as found in typical commercial buildings, or to the decontamination of unoccupied rooms, food storage facilities, or biosafety cabinets. Ozone has been previously investigated as an aerial disinfectant, but with inconclusive results (Hartman, 1925; Elford and Eude, 1942; Rodberg et al., 1991). The disinfection of entire rooms with ozone shows some promise (Masaoka et al., 1982), but data on ozone disinfection of air remain sparse. A summary of results obtained by previous investigators is shown in Table I for bacteria and in Table II for viruses. These results indicates that ozone can inactivate pathogens rapidly and efficiently in water. The possibility that similar results could be obtained in air has motivated this study. The toxicity of high ozone concentrations to humans presents an obvious obstacle to such applications, but the development of efficient ozone filters (Reiger et al., 1995; Takeuchi & Itoh, 1993) and ozone-destruction catalysts (Rodberg et al., 1991) offer some means of removing residual ozone and producing sterilized, breathable air. In addition, the low doses that have proven effective in water (Katzenelson et al., 1973; Hart et al., 1995; Beltran, 1995; Chang et al., 1996) coupled with the rapid decomposition rates that have been observed (Horvath et al., 1985, McCarthy and Smith, 1974; Harakeh and Butler, 1985), indicate that engineered alternatives to ozone filters may be feasible. These alternatives include ultraviolet irradiation and heating to enhance decomposition, extended residence time in mixing plenums, and the catalytic effect of glass and silica (Oeuderni et al., 1996).
A secondary application is the sterilization of surfaces or medical equipment with airborne concentrations of ozone. The use of UVGI and autoclaving typically require at least 20 minutes of exposure for complete sterilization of equipment. The use of ozone to sterilize equipment has the special advantage of both rapid inactivation and lower overall energy consumption. Applications could also include the sterilization of surfaces of contaminated rooms, biosafety cabinets, or entire buildings. Elimination of bacteria and viruses through ozonation should prove as effective in air as it does in water on a mass fraction basis, normalized to the mass of the fluid. Ozone does not react significantly with water or air in the absence of UV radiation over the short periods required for pathogen inactivation. These fluids merely provide the medium in which concentrations of ozone diffuse and react with organic molecules. Under ultraviolet irradiation, however, ozone reacts with water and decomposes into various short-lived radicals, such as the highly reactive hydroxyl radical. Theoretical and empirical evidence suggests that most of the sterilization effect results from the radicals produced, and not the ozone itself (Rice, 1997; Beltran, 1995). The decomposition reaction can be enhanced in air by the use of ultraviolet irradiation and through controlled humidity (NIST, 1992). Theoretically, therefore, the effects of ozone in air, under controlled conditions, should parallel the effects of ozone in water, and the effectiveness of ozone for eliminating airborne pathogens in either medium may be comparable.
The threshold concentrations at which ozone inactivates viruses and bacteria in water are remarkably low. For example, the threshold for Escherichia coli lies between 0.1 and 0.2 ppm (Katzenelson et al., 1973; Broadwater et al., 1974). Viruses are also sensitive to low levels of ozone, an advantage for an air-based system, since these small microbes are especially difficult to remove by filtration. With the Occupational Safety & Health Administration (OSHA) limit set at 0.1 ppm for human exposure, the design of an air-based ozonation system for indoor environments appears feasible. Figure 1. Diagram of experimental setup with 3D view of ozonation chamber, showing the dimensions, and the location of temperature and relative humidity sensors. 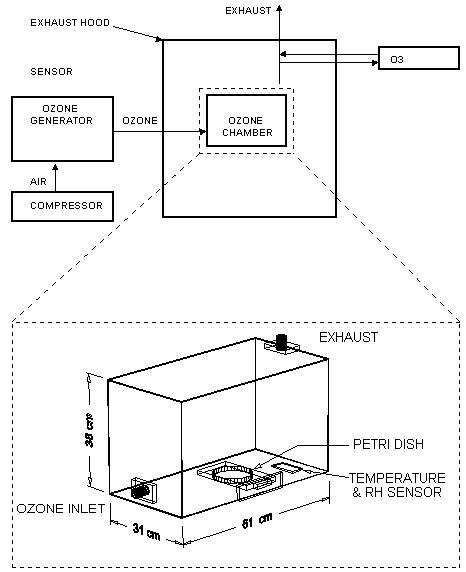
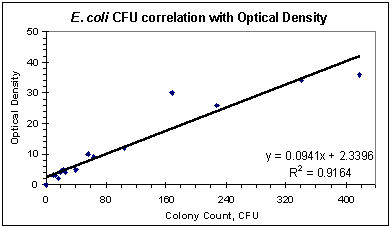
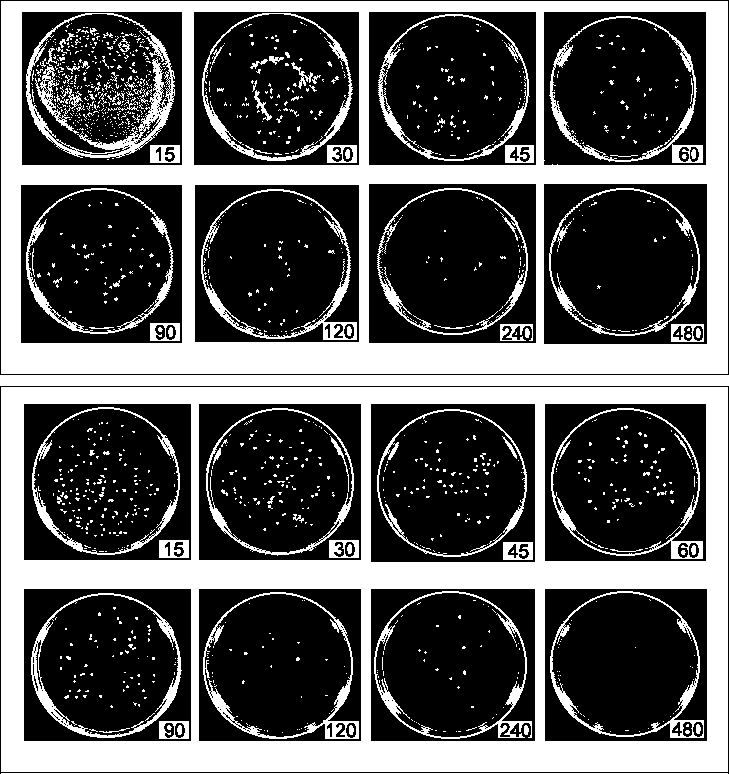
The purpose of the experiments described below was to determine the kinetics of disinfection for various concentrations of ozone in air. Two model bacterial organisms, E. coli and S. aureus, were used to assay a wide range of ozone concentrations and exposure times on killing rates. The results demonstrate that airborne ozone levels can effectively kill bacteria cells and sterilize surfaces. Material & Methods Bacterial cultures grown on solid media in Petri dishes were ozonated in an experimental ozone chamber of approximately 72 liters in volume (see Figure 1). An Oxytech corona-discharge ozone generator supplied a controlled amount of ozone to the chamber. The maximum flowrate to the chamber was 6.8 scfm, but the ozone generation rate was adjusted to maintain a constant ozone level in the chamber, as measured by a Dasibi ozone sensor. A compressor and ballast tank operating at 100 psig provided compressed air to drive the ozone generator, but the pressure in the ozone chamber was near ambient. Sensors mounted inside the chamber monitored air temperature and relative humidity, which remained between 23-24oC and 18-20% respectively, throughout the trials. The test chamber and sensor were placed inside an exhaust hood, which contained any ozone leakage. A magnetically sealed, sliding drawer in the test chamber allowed rapid insertion and removal of the bacterial samples without disturbing internal ozone concentrations. For this experiment, E. coli strain 528.10 and an S. aureus strain were selected to represent gram-negative and gram-positive species. Besides being commonly available, both species have often been used in experiments such as these and should therefore provide a familiar basis of reference. Cultures were grown overnight (for 18-20 hours) in Bacto Nutrient Broth (Difco Laboratories, Detroit, MI) by shaking at 37oC to concentrations of approximately 108 cells/ml, as verified by readings on a spectrometer (Milton-Roy Spectronic 1001). Approximately 0.1 ml of pure (or diluted) culture was spread on the surface of Petri dishes of Mueller-Hinton brown agar, that were prepared per the manufacturer's instructions. Control cultures were diluted to a titre of 104 cells/ml, while ozonated cultures were diluted to either 107 cells/ml or 106 cells/ml. Dilutions were made in two stages to minimize error: primary dilutions were prepared for the ozone trials first, then the control samples were made by diluting the primary dilutions 100-fold. After plating, the cultures were allowed to dry on the agar surface for approximately 2 hours. Control samples were individually inserted into the ozone chamber with continuous airflow but no ozone injection. The ozone generator was then engaged and adjusted until levels of ozone in the chamber stabilized at the desired concentration. The ozonated samples were individually inserted for intervals of 10 to 480 seconds, as timed by a digital stopwatch. Ozone levels were controlled to a constant level throughout each trial, typically within a limit of +/- 3%. Exposed cultures were then incubated for a period of 12 to 16 hours at 37oC, after which the plates were digitally imaged. The bacterial colonies were counted with an AlphaImager Digital Image Analysis System. Results were expressed in terms of colony forming units (CFUs). Counting the bacterial colonies was complicated by both the high rates of inactivation, which necessitated the use of dense surface concentrations, and by clumping effects. Clumped cells self-protect against the corrosive action of ozone, and tend to predominate after the first few seconds of exposure have eliminated most of the solitary cells. This effect made precise counts difficult in some cases, even when performed manually. Some of the plates could not be counted digitally or manually and were extrapolated based on optical density readings from contrast-enhanced images, produced with AlphaImager 2000 software. These digital images provided a well-defined outline of the bacterial cultures, and the optical density, or ratio of white area to black area, provided a reasonably accurate estimate of colony count. Correlations were determined by comparing manual counts with optical density estimates. High correlations (0.81 < r2 < 0.97) demonstrated that no serious error was introduced by this method. Figures 3a and 3b show some of the contrast-enhanced plates, of which only the first E. coli plate was uncountable by regular methods and was optically estimated. Figure 2a and 2b: Correlation of CFU count with optical density readings. Relation used to estimate CFUs for uncountable plates.
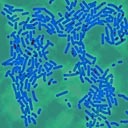
Figures 3a & 3b. Plates of Escherichia coli (top) and Staphylococcus aureus (bottom) exposed to ozone in air. The sequence of inactivation is shown with the exposure times given in seconds. These digital photos have been contrast enhanced to highlight the colonies against the agar background.
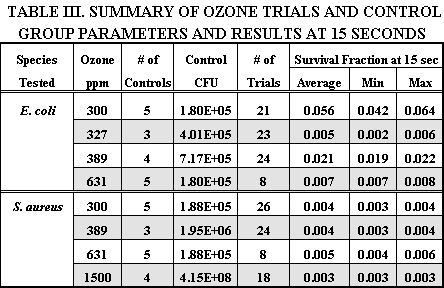
Results Table III summarizes the number of bacterial samples tested, the ozone concentrations used, and the survival fraction after 15 seconds of exposure. The survival rate is the arithmetic mean of the CFU remaining after ozone exposure. Survival rate was calculated as the ratio of the CFU of the exposed samples to the CFU of the control samples. The survival rates shown in the final column all represent dramatic reductions in CFU that accord with the previous results displayed in Tables I and II. Control groups exposed only to air in the chamber showed negligible reduction in CFU, regardless of exposure time. The survival rates have no relation to ozone concentrations, indicating the threshold of concentration dependence has been exceeded.
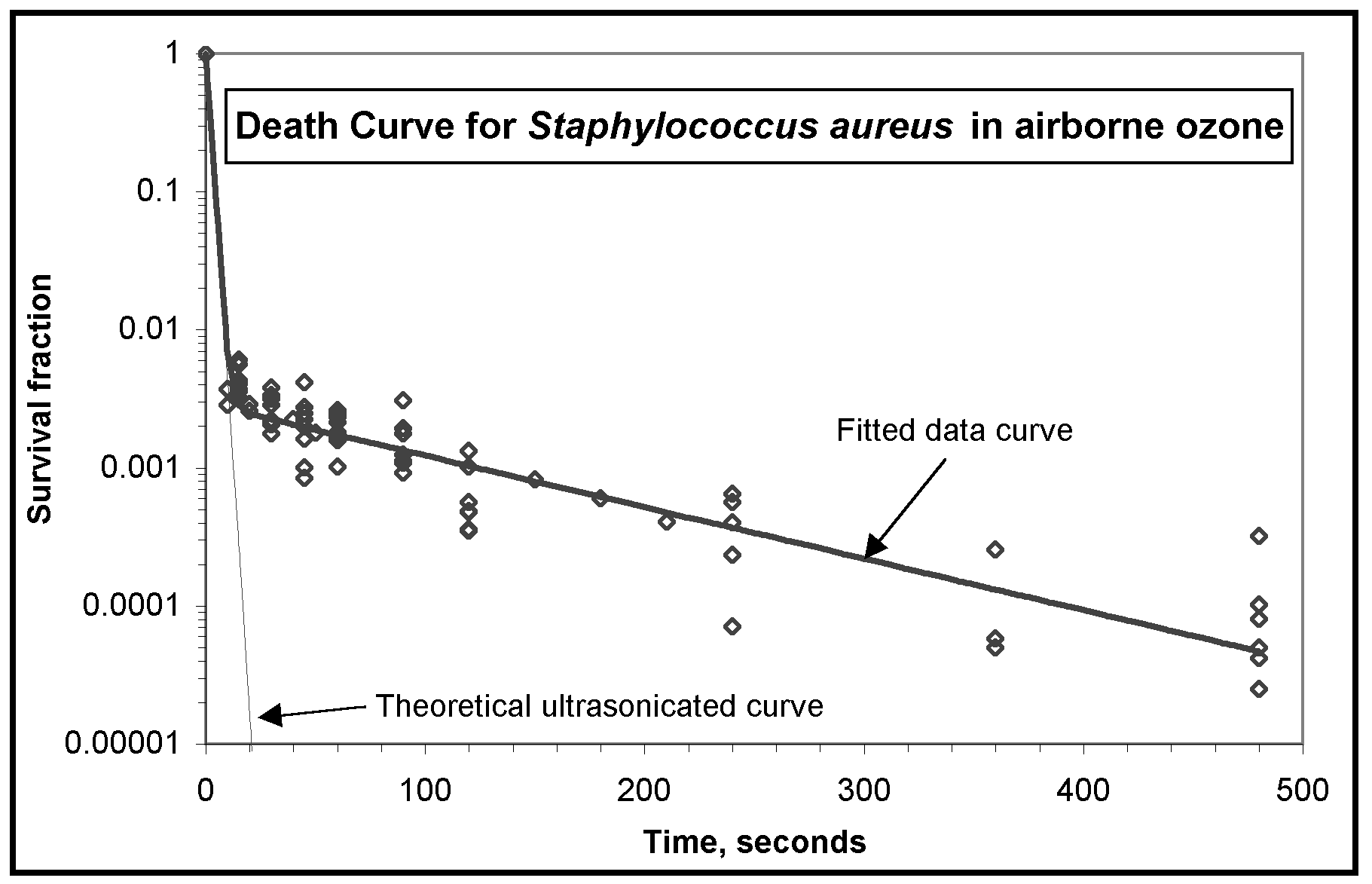
The sequence of inactivation from ozone exposure of between 15 to 480 seconds is illustrated in the contrast-enhanced images of Figure 3a and 3b. In a few cases, the cells were completely extinguished within 8 minutes, but most of the survival curves tailed off to near-zero levels. Data taken in these experimental trials have been combined to form the death curves presented in Figure 4a and 4b. The lack of concentration dependence (see Table III) allowed the data from different concentrations of ozone to be combined in the same graphs. Figure 4a and 4b. Death curves for Escherichia coli and Staphylococcus aureus in ozonated air. Ozone concentrations during this series of experiments varied from 300 to 1500 ppm.
Discussion The results for 15 seconds of exposure to airborne ozone shown in Table III compare well with the water-based results from other investigators shown in Table I and II. Compared with the three other airborne ozone studies (Elford, 1942; Heindel et al., 1993; de Mik, 1977), our results exhibit very comparable rates of inactivation, even though these earlier studies used much lower ozone concentrations. Also, some of the longer exposure times in Table I and II represent ozone concentrations below the threshold of maximum effectiveness. S. aureus cells demonstrated a higher initial sensitivity to ozone than E. coli but no significant difference could be observed between the survival tails beyond about 60 seconds. In fact, the strong similarity between the second stage decay rates suggests the resistance mechanism is not species-dependent (i.e. clumping). The effect of ozone on living cells is concentration-dependent up to a threshold specific for each species of virus or bacteria, and above which there is no increase in killing effect (Broadwater et al., 1974; Katzenelson and Shuval, 1973; McCarthy and Smith, 1974). Thresholds for various microbes are generally in the range of 0.1 - 0.2 ppm, but some researchers have found a weak concentration dependence up to about 10 - 12 ppm (Bunning and Hempel, 1995; Finch et al., 1988). The ozone concentrations we have used, 300 ppm and higher, precluded any concentration-dependence. This was confirmed by an absence of any concentration dependence in the range of 300 - 1500 ppm. The actual threshold could not be determined in this experiment because of equipment limitations, but most probably exists somewhere near 0.1 ppm in air, as it does in water. If complete sterilization can be achieved in air with such low concentrations, the use of ozone filters or other means of enhanced decomposition may be unnecessary. Each of the death curves shown in Figures 4a and 4b exhibit the characteristics of a two stage survival curve, where the first stage represents a rapid inactivation, and the second stage represents a small resistant fraction of the population. These logarithmic decay curves are in good agreement with the studies of viruses and bacteria by the previous investigators listed in Table III. The curves have a pronounced tailing-off effect, identical to those often noted but not analyzed in earlier studies (Cerf, 1977). The tailing of the survival curve indicates more than one rate constant is operating. Mathematically, this can be easily represented by the addition of two logarithmic functions, one with a rate constant representing rapid inactivation, and one with a much smaller rate constant representing extremely slow inactivation. A derivation of the basic inactivation equation will clarify this matter.
OZONE DISINFECTION KINETICS Empirical and theoretical studies (Chick, 1908; Davidovich and Kishchenko, 1991; Rahn, 1945) note that the survival S of a population dependent on some factor A is defined by an equation of the form
Ln [S] = -kt (1)
In the following derivation, we will attempt to demonstrate that the basis for a form of equation (1) lies in the chemical kinetics of ozonation. The idealized chemical reaction for ozone and any reactants is:
X + O3 ß à XO3 (2)
In this relation, X represents any carrier molecule, and XO3 represents any transitional product of the form XON, including hydroxyl radicals and others. The relation for the rate constant Kx, is
Kx =
The second stage reaction between the transitional product and the critical factor A can be idealized as follows:
XO3 + A ß à AON + XOM (4)
In equation (4), A may be any bacterial constituent or cell wall component on which bacterial survival is directly dependent. Again, the nomenclature is illustrative only, and the forms AON and XOM could represent many oxidation products. For the purpose of this derivation, they are all represented by a single value of k, the bulk average rate constant for the overall process. For a constant volume batch reactor, a mass balance on A defines the rate constant rA in terms of a general first order reaction:
rA = -
In our application the transitional product [XO3] must be included, and we have
-
which is second order, but pseudo first order since [XO3] remains constant.
Substituting for [XO3] from equation (3), we obtain
Rearranging and integrating,
where k = k Kx [X] [O3]. And
-
Substituting equation (9) into equation (8) and rearranging
Ln
Because the ozone concentration [O3] in this experiment is well above the threshold of concentration-dependence, it will remain effectively constant. Combining all constants, including [X] and [O3], yields the standard form
Or, since S depends directly on [A], S = e- k t (13)
However, two decay processes with different rate constants occur simultaneously: the rapid die-off of the individual bacterial cells and the slow death of the resistant or protectively clumped bacteria. These fast and slow reaction processes are effectively independent and can be added directly. In other words, the total population responds to ozonation as if there were two different species present and the total effect of the ozonation is simply the addition of the separate effects. The slow reaction component of the equation is of the same standard exponential form, but has a different rate constants, or S = e-mt (14)
Defining C as the fraction of the fast decay population, and noting that the sum of the fractional populations must equal 1, the complete form of the equation can be written:
S = C e- k t + (1-C) e-m t (15)
This equation provides improved accuracy over the traditional single component equation for any survival curve that possesses a tail. A least-squares curve fit of the data plotted in Figures 4a and 4b was performed to determine the rate constants separately for the fast decay (0-15 seconds) and slow decay (15-480 seconds) portions of the curves. The coefficient defining the fraction of the resistant population (C-1) was then determined by calculating the zero-intercept of the slow decay curve. Further precision was obtained by maximizing the r2 value of the overall equations through an exhaustive search. This process consisted of sweeping the coefficients and constants, in all their permutations, through ranges of about +/-10%. This yielded the following equations:
For E. coli S = 0.9976 e- 0.25 t + 0.0024 e-0.0073t (16)
S = 0.9971 e- 0.50 t + 0.0029 e-0.0086t (17)
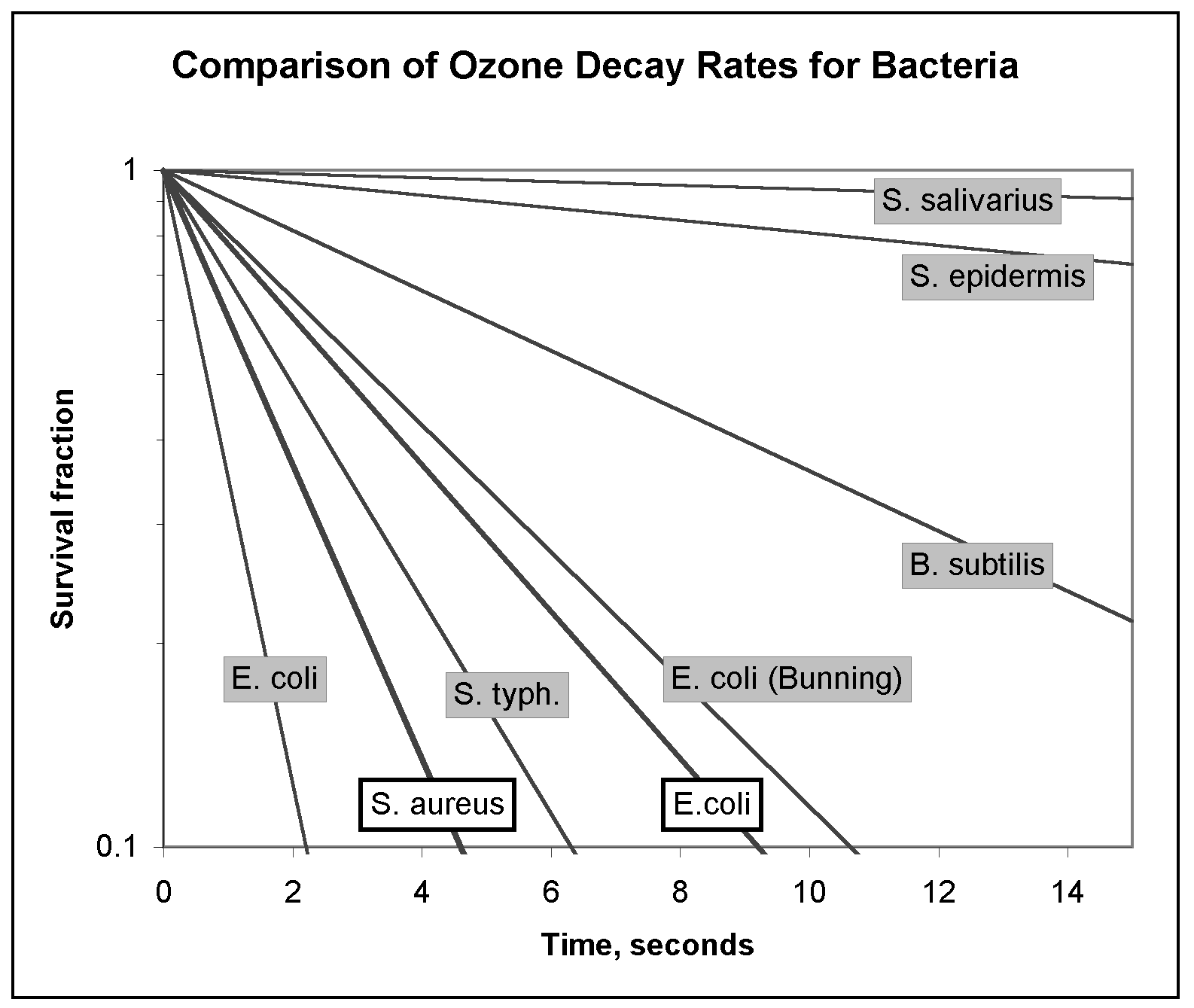
The overall least squares curve-fit of ln(S) in the above equations, and ln(S) of the data yielded r2 values of 0.85 and 0.83 for E. coli and S. aureus, respectively. In the curve fit for E. coli, logarithms of two data points representing zero survival at 480 seconds had to be ignored, for obvious mathematical reasons. This derived function, equation (15), permits a theoretical prediction of the effect of sonication, which will break up the clumps and make them vulnerable to ozone. Assuming that ultrasonication breaks up clumped cells without any significant inactivation, we can simply subtract the mathematical tail of the survival curve and examine the predicted theoretical survival rate. These values have been plotted as the dotted lines in Figures 4a and 4b, and are labeled the theoretical ultrasonicated curves. Inspection reveals that 99.999% destruction of E. coli or S. aureus might result in about 45 and 21 seconds respectively with combined ozonation and ultrasonication. These predicted values for ozone inactivation under ultrasonication are speculative, though, since they are heavily dependent on data from the first few seconds of ozone inactivation. Insufficient data points exist during these first few seconds because of the difficulties inherent in measuring results over short time periods. However, these estimates are likely to prove conservative; airborne pathogens will be exposed to ozone over their entire outer surfaces as opposed to those resting on surfaces with protected undersides. Figure 5 illustrates how the resulting decay rates determined in this experiment compare with those of previous researchers. The slope of the lines in Figure 5 represents the decay rate constant for the fast portion of the curve (the first stage). The decay rate for E. coli from Finch et al (1988) is exactly coincident with S. aureus and does not show. Figure 5. Comparison of decay rates obtained in this experiment (dark lines) with those obtained by previous investigators for ozonated bacteria (per Table I). Conclusions The results presented here demonstrate that high degrees of bacterial sterilization can be achieved with airborne ozone. The characteristics of the inactivation curves suggest strong parallels with ozonation of water-borne bacteria. The kill rates and exposure times in this experiment compare well with the best results obtained in numerous studies on the ozonation of pathogens in water. The results suggest that efficient sterilization of airstreams could be accomplished in a matter of seconds by the use of ozone. The most likely explanation for the tailing of the survival curves is the clumping effect suggested by various investigators – the tendency of micron-sized particles to clump naturally. Strong evidence for this phenomenon has been provided by combined ozone / sonication experiments (Katzenelson and Shuval, 1973; Katzenelson et al., 1985). The clumping of bacterial cells protects a small percentage of bacteria and causes them to behave as if they had a much higher resistance to ozone. Hypothetical extrapolation of these results to the ozonation of aerosolized bacteria would yield increased inactivation rates. Free-floating bacteria would not have a agar-protected underside, but would be exposed to ozone over their entire cell surface. Furthermore, the presence of moisture in the air, as humidity or in surrounding droplets, would tend to amplify the toxicity of ozone through the increased formation of radicals. If combined with natural or man-made UV irradiation, the production of toxic radicals could be maximized. The complete form of the equation describing the inactivation curve for ozonated pathogens has been developed from basic chemical theory. This equation provides an accurate fit of the data over the entire length of the survival curve, including the tail. This equation also provides a mathematical means of separating the behavior of clumped bacteria from solitary bacteria, and thus allows the prediction of the effects of sonication. The use of sonication may enhance the effect of ozonation in air as it does in water. The theoretical effect of sonication combined with ozonation in air has been predicted conservatively to yield 99.99% inactivation within at least 40-60 seconds of exposure. The extremely small size of viruses provides some defensive and transport advantages, but results from previous experiments listed in Table III indicate that viruses are at least as sensitive to ozone as the relatively large bacteria. The effectiveness of ozone in killing viruses could be expected to parallel the results obtained for viruses ozonated in water but this remains to be studied. Ultrasonication has been demonstrated to be effective in increasing the viricidal activity of ozone in water, and it could be expected to have a similar effect in air. The exact concentration threshold of ozone in air, the use of ultrasonication or other means to enhance the disinfection of air with ozone, and an effective means of removing residual ozone are issues that warrant further study. The effect of ozone on spores is unknown and has never been studied in air or in water. In spite of these unknowns, the results presented here suggest that the development of an ozonation system capable of sterilizing supply airstreams may be feasible in principle, and that the use of high levels of airborne ozone to sterilize surfaces or non-occupied rooms may be feasible in fact. Acknowledgments We are indebted to The Penn State Applied Research Laboratory, especially Janice Schneider and Brad Striebig, for loaning us the ozone equipment used in this experiment. Most sincere thanks also to Elizabeth McGraw, Eric Finch, and Sean Reid of the Biology Dept., Dr. Robert Kabel, Chemical Engineering, and Dr. John Cimbala, Mechanical Engineering, who assisted and supported us in this exploration. Thanks also to Michael Liberatori who made new brass fittings for us when all the rubber fittings on the ozone chamber suddenly disintegrated. References Ozone, Disinfection, Airborne Pathogens, Respiratory Infections, Microbial IAQ, Ultrasonication, Disinfection Kinetics, Survival Curves, Tailing, Clumping, E. coli, S. aureus, Surface Disinfection. |
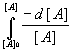 =
= 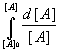 = - Ln
= - Ln