DCA
What is DCA
Sodium Dichloroacetate (DCA), is a chemical compound, known since 1864, that improves human energy production (mitochondrial function) and promotes cancer cells to recede and commit suicide (apoptosis).
DCA is a white powder that easily dissolves in water. It is essentially odourless and tasteless.
| Statements on this website have not been evaluated by the FDA,
TGA,etc and are not intended to diagnose, treat, cure or prevent
any disease. Always
consult with
your health care professional before undertaking any course of treatment.
We do not encourage anyone to take any of the abovementioned substances. DCA. DCA should be taken under medical supervision. Everything that is written here are subjective interpretation of data provided by the doctors from the University of Alberta and Medicor Cancer Centres, PUB MED and people;s personal experiences. If you are interested in learning more about DCA, please feel free to browse this webpage. This website serves educational purposes only, we are not doctors and can not provide medical advice for you |
.
;
Is DCA Safe?
is It safe to consume at least 12.5mg/kg of DCA a day.?
15 Month Treatment with DCA Revealed No Blood, Liver, Kidney or Heart
Toxicity.
We treated patients with a starting dose of 12.5 mg/kg orally twice a
day for 1 month, at which point the dose was increased to 25mg/kg orally
twice a day. We then followed a dose de-escalation protocol, decreasing
the dose by 50% when dose-limiting toxicity (temporary peripheral neuropathy)
occurred. The patients were followed clinically [and administered DCA]
for up to 15 months. None of the patients had hematologic, hepatic, renal,
or cardiac toxicity. Peripheral neuropathy was the only apparent toxicity.
Patients had variable dose-dependent degrees of peripheral neuropathy,
which was reversible, confirming previous studies. When the dose was
decreased to 6.25 mg/kg orally twice a day, none of the patients had
clinically significant peripheral neuropathy.
Department of Medicine, University of Alberta, Edmonton, Alberta, Canada.
Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E,
Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS,
Petruk KC.
Sci Transl Med. 2010 May 12;2(31):31ra34.
http://www.ncbi.nlm.nih.gov/pubmed/20463368
Download the Full Article in PDF forma
;
DCA Promotes Cancer Cell Suicide (apoptosis) and Inhibits
Cancer Growth
The unique metabolic profile of cancer (aerobic glycolysis) might confer
apoptosis resistance and be therapeutically targeted. Compared to normal
cells, several human cancers have high mitochondrial membrane potential
(DeltaPsim) and low expression of the K+ channel Kv1.5, both contributing
to apoptosis resistance. Dichloroacetate (DCA) inhibits mitochondrial
pyruvate dehydrogenase kinase (PDK), shifts metabolism from glycolysis
to glucose oxidation, decreases DeltaPsim, increases mitochondrial H2O2,
and activates Kv channels in all cancer, but not normal, cells; DCA upregulates
Kv1.5 by an NFAT1-dependent mechanism. DCA induces apoptosis, decreases
proliferation, and inhibits tumor growth, without apparent toxicity.
Department of Physiology, University of Alberta, Edmonton, AB T6G 2B7,
Canada.
Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson
R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K,
Porter CJ, Andrade MA, Thebaud B, Michelakis ED.
Cancer Cell. 2007 Jan;11(1):37-51.
http://www.ncbi.nlm.nih.gov/pubmed/17222789
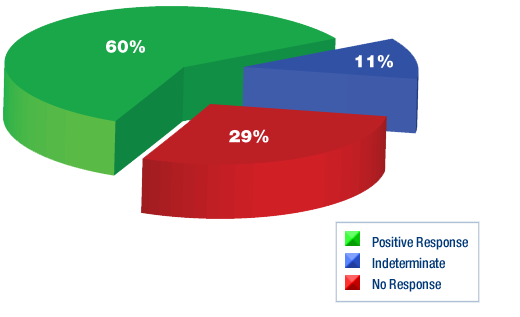
Five Patients with Brain Cancer Treated with DCA
Five patients who had brain cancer (glioblastoma) were treated with oral
DCA for up to 15 months. The dose-limiting toxicity was a dose-dependent,
reversible peripheral neuropathy, and there was no hematologic, hepatic,
renal, or cardiac toxicity. Indications of clinical efficacy were present
at a dose that did not cause peripheral neuropathy.
Patients 1 to 3 had already failed Radio Therapy and Chemotherapy and were considered terminal prior to starting DCA. Patients 4 and 5 were newly diagnosed.
•Patient 1: Before and after tissue samples showed decreased proliferation and increased cancer cell death (apoptosis). Also evidence of tumour regression seen by MRI scan.
• Patient 2: Cancer no longer visible on MRI or PET scan
• Patient 3: Before and after tissue samples showed decreased proliferation and increased cancer cell death (apoptosis). However this patient had a very large tumour, with brain edema at the start. They continued to deteriorate and died 3 months into the trial from brain edema.
• Patient 4: Before and after tissue samples showed decreased proliferation and increased cancer cell death (apoptosis). Also evidence of tumour regression seen by MRI scan.
•
Patient 5: No after tissue samples taken but evidence of tumour regression
seen by MRI scan.
All patients, except patient 3, were clinically stable at month 15 and
alive at a month 18 telephone follow-up.
Department of Medicine, University of Alberta, Edmonton, Alberta, Canada.
Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E,
Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS,
Petruk KC.
Sci Transl Med. 2010 May 12;2(31):31ra34.
http://www.ncbi.nlm.nih.gov/pubmed/20463368
Download the Full Article in PDF format
Type of Response to DCA Treatment
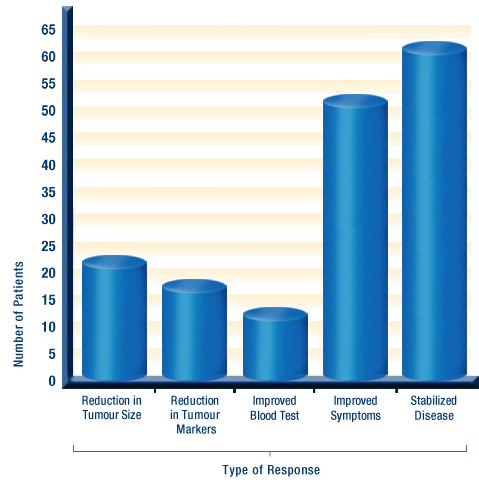
Their treatment regimen is typically 20-25mg/kg/day with a cycle of two weeks on DCA and one week off
The Pharmacology of DCA
Dichloroacetate (DCA) exerts multiple effects on pathways of intermediary
metabolism. It stimulates peripheral glucose utilization and inhibits
gluconeogeneis, thereby reducing hyperglycemia in animals and humans
with diabetes mellitus. It inhibits lipogenesis and cholesterolgenesis,
thereby decreasing circulating lipid and lipoprotein levels in short-term
studies in patients with acquired or hereditary disorders of lipoprotein
metabolism. By stimulating the activity of pyruvate dehydrogenase,
DCA facilitates oxidation of lactate and decreases morbidity in acquired
and congenital forms of lactic acidosis. The drug improves cardiac
output and left ventricular mechanical efficiency under conditions
of myocardial ischemia or failure, probably by facilitating myocardial
metabolism of carbohydrate and lactate as opposed to fat. DCA may also
enhance regional lactate removal and restoration of brain function
in experimental states of cerebral ischemia. DCA appears to inhibit
its own metabolism, which may influence the duration of its pharmacologic
actions and lead to toxicity. DCA can cause a reversible peripheral
neuropathy that may be related to thiamine (B1) deficiency and may
be ameliorated or prevented with thiamine supplementation.
Department of Medicine, University of Florida, College of Medicine,
Gainesville 32610.
Stacpoole PW.
Metabolism. 1989 Nov;38(11):1124-44.
http://www.ncbi.nlm.nih.gov/pubmed/2554095
DCA as Therapy for Blocked Arteries and Heart Failure
Short-term clinical and laboratory experiments demonstrate that intravenous
DCA rapidly stimulates pyruvate dehydrogenase enzyme complex activity
and, therefore, aerobic glucose oxidation in myocardial cells. Typically
these effects are associated with suppression of myocardial long chain
fatty acid metabolism and increased left ventricular stroke work and
cardiac output without changes in coronary blood flow or myocardial
oxygen consumption. Although long-term studies are lacking, short-term
parenteral administration of DCA appears to be safe and capable of
significantly improving myocardial function in conditions of limited
oxygen availability by increasing the efficient conversion of myocardial
substrate fuels into energy.
Sanger Clinic and the Department of Medicine, University of Florida
College of Medicine, Gainesville 32610, USA.
Bersin RM, Stacpoole PW.
Am Heart J. 1997 Nov;134(5 Pt 1):841-55.
http://www.ncbi.nlm.nih.gov/pubmed/9398096
We ship pure pharmaceutical
grade DCA, world wide,
after receipt of your order.
We do not take any responsibility for possible effects of taking DCA.
Should you wish to take personal responsibility and use DCA on your own, we insist that you consult your physician. You have to be aware of the fact that not all doctors accept DCA as a drug and it may be prescribed formally only in few countries.
 |
100 grams Pure DCA (3 months supply) |
|
Is DCA safe?
DCA has been used in humans to treat a rare disease called “congenital
lactic acidosis”, and found to have some mild to moderate side
effects. Our experience so far suggests that DCA is safe to use in cancer
patients under close medical supervision.
Some animal studies show that DCA can itself cause liver cancer. These studies used doses which are over 1000 times higher than what would be prescribed for cancer treatment.
We think that DCA can have 2 main categories of side effects:
Neurological:
Nerve injury in the hands and feet (“peripheral neuropathy”).
Neuropathy typically takes several weeks to months to develop, and is
reversible if it is caught early. In the existing literature, neuropathy
from DCA appears to be age-related. We suggest vitamin B1 (benfotiamine
or thiamine) and alpha lipoic acid to prevent and reduce the severity
of peripheral neuropathy.
Sedation, confusion, hallucinations, memory problems, hand tremors. These side effects appear to be dose-dependent, and age-dependent which is consistent with existing human research on DCA.
We use sustained release alpha lipoic acid to prevent/reduce these side effects. Patient feedback suggests that this supplement is effective.
Gastrointestinal:
Heartburn, nausea, vomiting, indigestion. These side effects may occur
with DCA,
Other Side Effects:
Some patients experience pain at the sites of their tumour(s) within
the first few days of starting DCA. This may be an indicator of the
effectiveness of DCA.
Most side effects reported so far have been mild or moderate. Patients experiencing moderate side effects are usually taken off DCA as a precaution. Most side effects typically resolve within days after stopping DCA. Neuropathy can take weeks or months to improve.
TLS (Tumour Lysis Syndrome)
This is a condition in which a large number of tumour cells are rapidly
killed, causing a sudden release of the contents of the dead cells
into the bloodstream. It can result in abnormal heart rhythms, and
kidney failure. A detailed reference article can be found here. TLS
occurs most commonly in patients with a large mass of tumour cells
in the body who receive chemotherapy, especially with lymphomas or
acute leukemia. Since DCA can enhance the effect of chemotherapy
in certain cases, it may be more likely to occur if DCA is combined
with chemotherapy (especially without medical supervision).
DCA-Drug Interactions
Drugs that can cause confusion or hallucinations
have a potential to interact with DCA. This may include cannabinoids,
benzodiazepines and other CNS drugs. Patients NEED to consult
their physicians for potential drug interactions.
DCA and Caffeine
We have received a large number of inquiries about caffeine following
some anecdotal reports of enhanced DCA effect with excessive tea/caffeine
intake. After conducting a limited review of our DCA patients, we have
noted that patients who have shown an excellent response to DCA do
not take tea/coffee or caffeine or take it in minimal amounts (1-3
cups a day). Also a few patients with high tea/caffeine consumption
(5- 10 cups per day) have shown no response to DCA.
There are a number of potential harmful effects of starting high dose caffeine including increased likelihood of seizures in brain tumour patients, abnormal heart rhythms, anxiety, and insomnia.
DCA and Chemotherapy
Until now, there has been no scientific evidence to support the use of
DCA along with chemotherapy. For the first time in North America, Medicor and ORT are now conducting ChemoFit tests with DCA and chemo combined!
What this means is eligible patients can have a sample of their own tumor
analyzed to see if combinations of DCA and chemo will work, and if they
will work better than chemo or DCA alone. Click here to see a sample
ChemoFit test report with DCA. The accuracy of the ChemoFit test ranges
from 85-99%.
We have already had some exciting results showing that DCA can, in some cases, dramatically enhance the cancer-killing effects of chemo. However, there is a possibility that DCA can antagonize chemo as well. This is similar to single agent chemo being better than combination chemo for some patients. If you are a patient who is thinking of combining DCA and chemotherapy, we recommend you review our ChemoFit web page here and discuss the test with your oncologist. We also have more detailed information for physicians here.
Malignant ascites fluid samples and malignant pleural effusion samples can now be tested with ChemoFit, eliminating the need for a biopsy in some patients.
What is the status of DCA clinical trials?
Two DCA clinical trials have begun in Alberta. One will examine the effects
of DCA on a type of brain tumour (50 patients), and the other will
look at patients with various cancers (35 patients). If you are in
the Edmonton area, we recommend you consider enrolling in one of the
clinical trials. We have no further information about these trials.
Even though we have seen conclusive evidence of DCA’s effectiveness in various cancers, Medicor believes that it is essential for DCA clinical trials to be conducted. DCA is different from other drugs that undergo clinical trials because it is not a “new” drug. It has already been used for decades in humans, and has a relatively safe profile.
This means that the trials may take less time, but may still take years. Many cancer patients cannot wait this length of time. We are hopeful that information obtained from our experiences with DCA will supplement clinical trials, and help patients and the medical community.
Can I take DCA on my own?
We are aware of many patients who are currently self-medicating with
DCA. Due to the complexity of cancer and its treatment, we recommend
DCA to be taken under medical supervision.
Note: With regards to cancer, DCA seems not to work with sarcomas
Disclaimer
Altered States ltd and subsidaries makes material available on the
understanding that people exercise their own skill and care with respect
to its use. Before relying on the material in any important matter,
people should carefully evaluate the accuracy, completeness and relevance
of the information for their purposes and should obtain appropriate
professional advice relevant to their particular circumstances.
The material on this site may include the views or recommendations of third parties, which do not necessarily reflect the views of Altered States ltd or indicate its commitment to a particular course of action.
By accessing information at or through this site users waive and release Altered States ltd and its servants to the full extent permitted by law from any and all claims relating to the usage of the material made available through the website. In no event shall Altered States ltd be liable for any incident or consequential damages resulting from use of the material.
Altered States ltd is not responsible for the contents or reliability of the linked websites. We cannot guarantee that these links will work all of the time. These external information sources are outside our control.
 |
100 grams Pure DCA
(3 months supply)
|
|